In recent years, the development of new and powerful organocatalytic cascade reactions has emerged as an area of considerable interest. This is because of the advance in the different activation modes in organocatalyisis and the easy prediction of the stereochemical outcome of the reactions. Using organocatalysis we can be able to synthesize very complex scaffolds in one pot reaction bearing several stereocenters and in enantiopure fashion.
.
In the past few years, our research group has been working on the development of new organocatalytic methodologies for the enantioselective construction of highly complex scaffolds. During our works, we become fascinated by the synthesis of spirocompounds. We applied our knowledge on organocascade reactions in the development of a highly stereoselective synthesis of spirolactames [1] or spirooxindoles and spiropyrazolones via a Michael-Michael-Aldol reaction.[3]
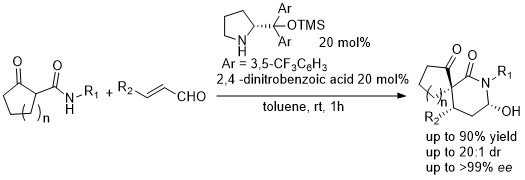
Figure 1: Organocatalytic cascade reaction for the synthesis of spirolactams
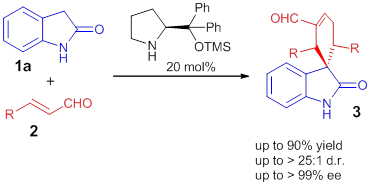
Figure 2 : Organocatalytic cascade reaction for the synthesis of spirooxindoles
We also have developed a new organocascade for the synthesis of alpha-methylene lactones (highlighted in Synfacts).

Related Publications:
1 “Highly diastereo and enantioselective synthesis of a-spiro-g-lactams by an organocascade reaction” Kaiheng Zhang, Marta Meazza, Vojtech Doceka, Mark E. Light, Jan Vesely, and Ramon Rios* Eur. J. Org. Chem. 2017, 1749-1756. (Highlighted in Organic Chemistry Highlights webpage).
2 “Enantioselective Organocatalytic Cyclopropanation of Enals using Benzyl Chlorides” Maria Ashe, Marta Meazza, Hun Yi Shin, Hye Sung Yang, Andrea Mazzanti, Jung Woon Yang,* and Ramon Rios,* J. Org. Chem. 2016, 81, 3488-3500.
3. "Organocatalytic synthesis of spiro compounds via a cascade Michael-Michael-Aldol reaction" X. Companyó, A. Zea, A.- N. R. Alba, A. Mazzanti, A. Moyano and R. Rios*. Chem. Commun. 2010, 46, 6953-6955.
4. "Highly stereoselective synthesis of spiropyrazolones" A. Zea, A.-N. R. Alba, G. Valero, T. Calbet, M. Font-Bardía, A. Moyano, R. Rios*. Eur. J. Org. Chem. 2011, 1318-1334.
5. “Highly enantioselective cascade synthesis of spiropyrazolones” A. Zea, A.-N. R. Alba, A. Mazzanti, A. Moyano, R. Rios*, Org. Biomol. Chem. 2011, 9, 6519-6523.
6- “Expanding the scope of organocatalytic monofluorination of enals. Enantioselective synthesis of fluoroindanes and fluorochromanols” B. Wang, Y.-S. Kim, S. Kim, X. Companyó, J. W. Yang, Jing Li , A. Moyano, R. Rios, Adv. Synth. Catal. 2014,356, 437-446
7- “One-pot organocatalytic synthesis of a-methylene-g-lactams”, Xavier Companyó, P. Geant, A. Moyano, A. Mazzanti, A. Janecka, R. Rios* Tetrahedron, 2014, 70, 75-82.
8- “First One-pot organocatalytic synthesis of a-methylene-g-lactones” Xavier Companyó, A. Moyano, A. Mazzanti, A. Janecka, R. Rios* Chem. Commun. 2013, 49(12), 1184-1186. Highlighted in Synfacts (Synfacts; 02, 2013)
9- “Highly diastreoseletive synthesis of piropyrazolones” Victor Ceban, Temitope O. Olomola, Marta Meazza, Ramon Rios* Molecules 2015, 20, 8574-8582.

