Organocatalysis is commonly accepted as the use of small organic molecules to catalyze organic transformations. The term “organocatalysis” was coined by David W. C. MacMillan at the beginning of the 21st century, and was the starting line for breathtaking progress in this area over the last decade. During these last years, this area has grown into one of the three pillars of asymmetric catalysis, complementing and sometimes improving bio- and metal catalysis. The rapid growth in this area can be easily explained: the field offers several advantages to researchers in academia and industry, such as easy and low cost reactions, and reactions that are insensitive to air or moisture (unlike organometallic chemistry). Furthermore, the small chiral organic molecules used as catalysts can be often be derived from nature; thus, they are accessible and inexpensive to prepare, and often, the processes are environmentally friendly. Moreover, the need in industrial large-scale production for removal of impurities related to toxic metal catalysts from the waste stream, which has a huge financial impact, could be avoided with the use of organocatalysts and this has made the field very interesting from the industrial point of view.
The renaissance of organocatalysis was at the beginning of the 21st century, but the origins of small organic molecules acting as catalysts can be traced back to the earliest works of Emil Knoevenagel. In these works, Knoevenagel studied the use of primary and secondary amines, as well as their salts as catalysts for the aldol condensation of b-ketoesters or malonates with aldehydes or ketones. Knoevenagel also suggested the same intermediates that Westheimer later proposed in his retro-aldolization studies. Another key development in the history of organocatalysis was the work of Dakin in 1910 regarding the catalytic activity of primary amino acids in the Knoevenagel reaction. Twenty years later, secondary amines were found by Kuhn and Hoffer that catalyzed not only the Knoevenagel reaction but also the aldol reactions between aldehydes. Our research group has been devoted to the development of new asymmetric methodologies for the synthesis of enantiopure compounds based in organocatalysis. We focus our interest in the synthesis of interesting targets like quaternary amino acids by organocatalyzed addition of azlactones to vinylsulfones, or in a general context the study of the oxazolone reactivity or MBH carbonate reactivity.
Recently, we focus our attention in the development of new organocatalytic methodologies for the synthesis of chiral BODIPYS. Those excellent fluorescents could be used as enantioselective probes in a variety of experiments with biological and chemical interest.[1]
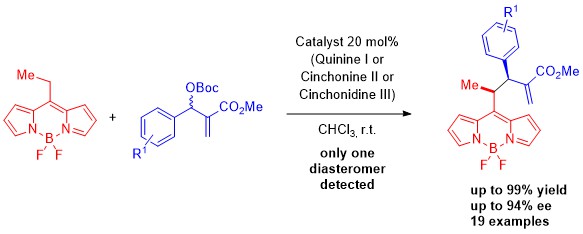
Figure 1: Organocatalytic addition of BODIPYs to MBH carbonates
We also have developed new enantioselective methodologies for the synthesis of g-allylic alkylation of quinolines [2]. Taking advantage of the reactivity of 2-allylquinolines we designed an addition to enals catalysed by secondary amines with excellent results.
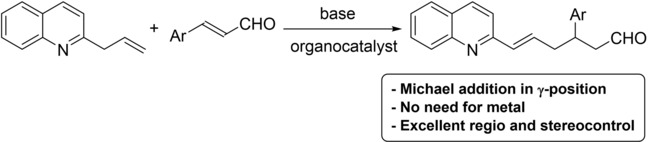
Figure 2 : Organocatalytic addition of 2-allylquinolines to enals
Examples of other methodologies sone in our reserach group [3-9]:
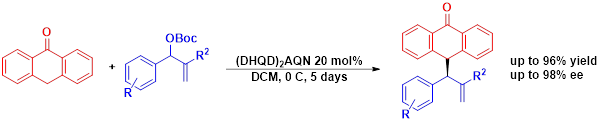
Figure 1: Organocatalytic addition of anthrones to MBH carbonates
1 “Studying the reactivity of alkyl substituted BODIPY. First enantioselective addition of BODIPY to MBH carbonates” Marta Meazza, Carlos M. Cruz, Ana Maria Ortuno, Juan Manuel Cuerva, Luis Crovetto,* Ramon Rios*, Chem. Sci. 2021, DOI:10.1039/D0SC06574A
2 “Highly Regio‐ and Enantioselective Organocatalytic γ‐Allylic Alkylation of Quinolines” Marta Meazza*, Ramon Rios, Adv. Synth. Catal. 2021, DOI:10.1002/adsc.202001213
3 “Organocatalytic Amination of Pyrazolones with Azodicarboxylates: Scope and Limitations” Bedhich Formnanek, Vit Seferna, Marta Meazza, Ramon Rios*, Mahendra Patil*, Jan Vesely* Eur. J. Org. Chem. 2021, DOI:10.1002/ejoc.202100167
4 “Highly Diastereo- and Enantioselective synthesis of a-spiro-d-lactams by an Organocascade Reaction” Kaiheng Zhang, Marta Meazza, Vohtech Docekal, Mark E.Light, Jan Vesely, Ramon Rios* Eur. J. Org. Chem. 2017, 1749-1756. (Highlighted in Organic Chemistry Highlights webpage)
5 “Highly enantioselective synthesis of alkylazaarenes derivatives through a Michael-Michael-Aldol cascade reaction” Marta Meazza, Michael Potter, and Ramon Rios* Eur. J. Org. Chem. 2017, 719-725.
6- “Enantioselective Organocatalytic Addition of Oxazolones to 1,1-Bis(phenylsulfonyl)ethylene: A Convenient Asymmetric Synthesis of Quaternary alpha-Amino Acids”. A.-N. R. Alba, X. Companyó, G. Valero, A. Moyano, R. Rios. Chem. Eur. J. 2010, 16, 5354-5361
7- "Enantioselective organocatalytic addition of azlactones to maleimides: A highly stereocontrolled entry to 2,2-disubstituted-2H-oxazol-5-ones" A.-N. R. Alba, G. Valero, T. Calvet, M. Font-Bardia, A. Moyano*, R. Rios*. Chem. Eur. J. 2010, 16, 9884-9889.
8- “Formal highly enantioselective organocatalytic addition of alkyl anions to a,ß-unsaturated aldehydes. Aplication to the synthesis of isotope-enantiomers”. A.-N. Alba, X. Companyó, A. Moyano, R. Rios. Chem. Eur. J. 2009, 15, 11095-11099.
9- “Expanding the scope of Metal-Free enantioselective allylic substitutions: Anthrones” Victor Ceban, Jiří Tauchman, Marta Meazza, Greg Gallagher, Mark E. Light, Ivana Gergelitsová, Jan Veselý* and Ramon Rios* Sci. Rep. 2015, 5, 16886.

